 Discovery Information Discovery Information
|
| Who: A. Ghiorso, Nurmia, Harris, K.A.Y. Eskola, and P.L. Eskola
|
| When: 1969 |
| Where: Berkeley California |
|
 Name Origin Name Origin
|
| In honor of Ernest R. Rutherford, a New Zealand physicist. |
| "Rutherfordium" in different languages. |
|
 Sources Sources
|
| Bombarding plutonium with accelerated 113 to 115 MeV Neon ions. Also by bombarding a target of Cf249 with C12 nuclei of 71 MeV, and C13 nuclei of 69 MeV.
|
|
|
 Uses Uses
|
| It has no uses. |
|
 History History
|
| Rutherfordium (named in honour of noted New Zealand nuclear physicist Ernest Rutherford) was reportedly first synthesized in 1964 at the Joint Nuclear Research Institute at
Dubna (U.S.S.R.). Researchers there bombarded 242Pu with accelerated 113 to 115 MeV 22Ne ions and claimed that they detected nuclear fission tracks in a special type of glass with a microscope which indicated the presence
of a new element.
|
| In 1969 researchers at the University of California, Berkeley synthesized the element by subjecting 249Cf and 12C to high energy collisions. The UC group also stated that they could not reproduce the earlier synthesis by Soviet scientists.
|
| This resulted in an element naming controversy; since the Soviets claimed that it was first detected in Dubna, dubnium (Db)
was suggested, as was kurchatovium for element 104, in honor of Igor Vasilevich Kurchatov (1903-1960), former head of Soviet
nuclear research. The Americans, however, proposed rutherfordium (Rf) for the new element to honour Ernest Rutherford, who
is known as the "father" of nuclear physics. The International Union of Pure and Applied Chemistry (IUPAC) adopted unnilquadium
as a temporary, systematic element name, derived from the Latin names for digits 1, 0, and 4. However in 1997 they resolved
the dispute and adopted the current name. (Element 105 was named Dubnium, instead.)
|
|
 Notes Notes
|
| Evidence of element 104 was first detected at the Joint Nuclear Research Institute at Dubna (USSR) in 1964 by bombarding plutonium with accelerated 113 to 115 MeV neon ions. By measuring fission tracks in a special glass with a microscope, the scientists detected an isotope that decays by spontaneous fission. The isotope was thought to be Rf260 with a half life of 0.15 to 0.3 seconds. It was not until 1969, however that the group in Berkley were able to chemically
separate element 104 and positively identified two possibly three isotopes of the element.
|
| In August of 1997 the International Union of Pure and Applied Chemistry announced the official naming of this element as Rutherfordium
with the atomic symbol of Rf. The IUPAC choose Rutherfordium over the Russians' choice of Kurchatovium, which was in honor
of Igor Vasilevich Kurchatov (1903-1960), former Head of Soviet Nuclear Research.
|
| Element 104 was previously known as Unnilquadium; from the latin from "one zero four". |
|




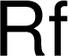
 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Uses
Uses
 History
History
 Notes
Notes